7 - Panels (Batteries)
This section last updated: 2021-06-01 (4 years ago)
Beginning with version 1.0, the LOINC database was expanded to include order sets/panels. We use the word “panel” to mean collector terms that contain links to an enumerated set of discrete child elements. Across domains, this generic concept of a collection might be called a battery, form, data set, etc. The same general panel structure in LOINC is used to represent all of these kinds of collections.
7.1 Goals
Our work in creating panels was stimulated by many requests for a standard set of test order codes from medical information system vendors who want to install their systems with a usable starter set of standard codes for common orders. They also want standard codes to ease the cross communications among merging hospitals.
LOINC codes have been defined for most individual laboratory observations and for many clinical observations, and claims attachments. The same LOINC codes can be used to order individual laboratory and clinical observations, as well as to report the result. For example, a LOINC code for Blood Hemoglobin (LOINC: 718-7) could be used as easily to order the test as to report its result. In HL7 messages, the field where the code is used indicates its role as an order (e.g. OBR-4) or a result observation (OBX-3).
Similarly, pre-existing LOINC codes could also be used to order more complex observations. The LOINC code used to order Urinary Creatinine Clearance (LOINC: 2164-2) actually implies an order for two distinct measures (serum creatinine and 24-hour urine creatinine) that are used to calculate the creatinine clearance.
However, the existing single value LOINC codes could not be used to order many laboratory and clinical procedures that are ordered as a single-named test (battery), such as CBC, urine dipsticks, blood differential count, and LDH isoenzymes. Similarly physicians order blood pressure measures and expect to get (at least) the diastolic blood pressure and the systolic blood pressure. Though these are separate observations, for practical purposes one is never measured without the other.
Initially, we created LOINC codes for the common “fixed” packages of observations. By fixed, we mean that certain kinds of measures will always be part of the battery, and the production of that particular set of measurements is tightly bound to the procedure, instruments that produce the values, or by a government mandate (e.g., LOINC: 24325-3: Hepatic function HCFA 2000 panel).
Over time, we have evolved our approach to building panels of other observation collections. (For information on using the ORDER_OBS field of the LOINC database to help find panels that could be used as orders, see Section 10.3.)
7.2 Types of results found in panels
To understand the rules about how LOINC creates panels and how to map your local panels to LOINC panels, it is important to distinguish among several kinds of “results”.
7.2.1 Primary measurements or observations
These observations report key measured results, and may indicate the presence/absence or amount of a substance or organism in the sample. For example, in an electrolytes panel, measured amounts of sodium, potassium, chloride, and bicarbonate would be the primary measures. Note that if a single primary result is being reported, the code for that result should be used for the order rather than a panel code. See Section 12.1.11 for more information.
7.2.2 Derived observations
These observations are derived from mathematical or logical operations on the primary measurements. For example, in an electrolytes panel, the anion gap is a derived measure that is calculated by subtracting the concentrations of chloride and bicarbonate (anions) from the concentrations of sodium and potassium (cations).
7.2.3 Ask at order entry (AOE) questions and other associated observations
These observations are obtained from the requester as part of the test order and are generally delivered back to the requester as part of the result package. For example, the concentration of inspired oxygen is always an AOE question for blood gas measurements. Similarly, the date of last menstrual period is an AOE question for pap smears.
Other kinds of observations are operationally treated in a similar manner although they may not be “questions” in the literal sense. For example, the volume and times (start, stop, duration) of urine collection are often sent along with the order and back again with the results. We call these “associated observations” and consider them like AOEs for the purpose of this discussion.
7.2.4 Impressions, interpretations, and comments
LOINC defines an impression/interpretation as a summary statement about multiple observations. This kind of summary observation is often included in collections of results sent back to the requester. (Such summary observation terms in LOINC have the Property of Imp, for impression, in their name).
As an aside, we note that decisions about test results being outside of normal range are best reported with the Interpretation Code (commonly referred to as the “Abnormal flag”) field of the HL7 OBX structure and not as a separate interpretation observation.
7.3 LOINC rules for panel names
We use most of the same general LOINC naming rules for panels as we do for individual observations. Here we describe some of the key unique features of panel names.
7.3.1 Component
If a government authority recognizes the panel or order set, we will name such panels with the year that the recognition took effect. For example, LOINC includes the “Comprehensive metabolic 2000 panel”.
For panels consisting of more than three constituent tests, the Component name will be a concatenation of:
- A name (e.g., Hemogram, Differential count, Vital Signs) to convey the content of the panel
- The word “Panel” included to unambiguously identify that this LOINC term refers to a panel or battery
In the case that a well-defined panel exists but has no conventional name or the panel consists of three or less constituents, we will include each of the distinct measured entities separated by ampersand (&) in the Component. We may also use a more efficient syntax that implies a repeat of the first part of the name, e.g., Chlamydia Ab IgM & IgG Panel.
Historically, LOINC created different codes for panels that contain reflex testing versus those that do not. In May 2020, the Lab LOINC committee decided that going forward, panel names would no longer specify reflex testing. Existing panels that specify reflex testing will be reviewed and updated as appropriate.
7.3.2 Property and Scale
Because the Property typically varies across the elements of a panel, the Property (i.e. the second part of the LOINC name) for the panel term may be populated by a dash (-). Likewise, the Scale (5th part of the LOINC name) will be populated by a dash (-) if the panel elements contain different scales.
7.3.3 Time Aspect and System
Because these parts of the LOINC name are typically consistent across the primary measures of a panel, the Time Aspect and System of a panel name are usually specified with the same conventions that apply to observation terms.
7.3.4 Examples of LOINC Panel names
The following are a few examples of LOINC Panels (Order Set Names):
| LOINC_NUM | LOINC Fully Specified Name | Description |
|---|---|---|
| 24358-4 | Hemogram WO platelets panel:-:Pt:Bld:Qn | HCT & HGB & WBC & RBC & Indices |
| 24359-2 | Hemogram WO platelets & W manual differential panel :-:Pt:Bld:Qn | Hemogram & Differential Count |
| 24317-0 | Hemogram & platelets WO differential panel:-:Pt:Bld:Qn | HCT & HGB & WBC & RBC & Indices & Platelets |
| 24338-6 | Gas panel:-:Pt:Bld:Qn | pH & PO2 & PCO2 on blood without specifying whether arterial, venous, or other source. The report would usually include an observation about the inspired O2 sent along with the report. It may include a variety of other patient characteristics sent by the requester and a variety of computed variables. |
| 24336-0 | Gas panel:-:Pt:BldA: Qn | pH & PO2 & PCO2 on arterial blood. The report would usually include an observation about the inspired O2 sent along with the report. It may include a variety of other patient characteristics sent by the requester and a variety of computed variables. |
| 24339-4 | Gas panel:-:Pt:BldV:Qn | pH & PO2 & PCO2 on venous blood. The report would usually include an observation about the inspired O2 sent along with the report. It may include a variety of other patient characteristics sent by the requester and a variety of computed variables. |
| 29274-8 | Vital signs measurement:Find:Pt:^Patient^Multi | Diastolic Blood Pressure & Systolic Blood Pressure & Pulse Rate & Respiratory Rate |
| 24357-6 | Urinalysis macro (dipstick) panel:-:Pt:Urine:- | Urinalysis dipstick results. Usually includes Glucose, Bilirubin, estimate of leukocytes, estimate of RBCs, estimate of bacteria, Ph, Specific gravity. But we do not make distinctions about the exact set of measures on the dipstick. The ordering clinician will not necessarily know what particular dipstick is being used and is not able or interested in making those distinctions. |
| 29576-6 | Bacterial susceptibility panel:-:Pt:Isolate:OrdQn | Would include susceptibility results for the antibiotics relevant to the isolates and the kind of culture. |
7.4 Representing conditionality
Within the LOINC panel structure, we can set an attribute for each element of the panel that denotes whether or not it should be present (conditionality) in the panel when resulted. The primary measures are always required, but the other elements of the panel may have different kinds of conditionality.
The conditionality attribute is visible on the details pages for panel terms under the column “R/O/C” and stored in the ObservationRequiredInPanel field of the LOINC panels and forms file (available for download from the LOINC website).
Through version 2.48 of the LOINC database, the conditionality choices were limited to R (Required), O (Optional) and C (Conditional). Example of simple order set with terms that are either R or O:
| 24358-4 | Hemogram WO platelets panel | - | Pt | Bld | Qn | R/O/C |
|---|---|---|---|---|---|---|
| 26464-8 | Leukocytes | NCnc | Pt | Bld | Qn | R |
| 26453-1 | Erythrocytes | NCnc | Pt | Bld | Qn | R |
| 718-7 | Hemoglobin | MCnc | Pt | Bld | Qn | R |
| 20570-8 | Hematocrit | VFr | Pt | Bld | Qn | R |
| 30428-7 | Mean corpuscular volume | EntVol | Pt | RBC | Qn | R |
| 28539-5 | Erythrocyte mean corpuscular hemoglobin | EntMass | Pt | RBC | Qn | R |
| 28540-3 | Erythrocyte mean hemoglobin concentration | MCnc | Pt | RBC | Qn | R |
| 30384-2 | Erythrocyte distribution width | EntVol | Pt | RBC | Qn | O |
| 30385-9 | Erythrocyte distribution width | Ratio | Pt | RBC | Qn | O |
| 76069-4 | Erythrocytes.hypochromic/100 erythrocytes | NFr | Pt | Bld | Qn | O |
| 76090-0 | Erythrocytes.hyperchromic/100 erythrocytes | NFr | Pt | Bld | Qn | O |
Starting with version 2.50 (December 2014), we expanded the choices beyond R, O and C to include R-a (Required with alternative), Rflx (Reflex) and Rflx-a (Reflex with alternative). The expanded choices better accommodate reflex tests, cases in which more than one test could be used to fulfill a given requirement of the panel, and focus the use of C (which had previously been used as a “catch-all” for many types).
The following table describes the expanded set of conditionality choices.
Table 29: Options for Term Conditionality within Panels
| Code | Meaning | Discussion /Definition |
|---|---|---|
| R | Required | Means this test must be included. |
| R-a | Required with alternatives | Means required but has alternatives. The alternatives will usually be paired, and both will be marked with the R-a code. Results for at least one member of the pair must __be included in the panel to satisfy the required condition, but both may be included. The C, conditional, had been used to deal with these cases in the past. The rule for pairing up of the alternatives for a given purpose will be described in a narrative usually in the panel description because the same rule will apply to many pairings in the panel. For example, the LA panel includes lots of coagulation measures and almost all can be reported as an actual time and/or as the ratio of patient time to the normal time. Measures of the same clotting function that differ only by how they are reported would be considered paired alternatives in this panel. More specific pairing rules may also be asserted in the “condition for inclusion” field associated with each item in the panel. |
| C | Conditional | Means that the required status of a given variable depends on other factors. This was too much of a waste basket category, which is why we added some new categories to specify what had usually been lumped as conditional. |
| O | Optional | Means the variable may be included or not, but whether it is included or not has no bearing on the whether a given set of result represents the given panel. Simple calculations derived from other reported measurements and ask at order entry questions are almost always optional. |
| Rflx | Reflex | Means this test will be included in the panel only if it satisfies a reflex condition based on other results in the panel. Such cases had also been lumped under the conditional code in the past. In general, we will not define specific rules for reflexing except in very general terms. |
| Rflx-a | Reflex with alternatives | Means that that the test is a reflex but that another test in the panel can serve as an alternate and either or both of these reflex tests can be reported. But if the reflex condition is met, at least one of the alternative pairs must be included. As in the case of R-a, what tests pair up as alternatives will be defined in a narrative in the panel description of the condition for inclusion field. |
If more than one measure can serve as a primary measurement, then both of them will be required with alternatives (R-a), and the criteria is that at least one of them must be present. An example would be the absolute count of neutrophils versus the percentage of total white count of neutrophils in a differential blood count – if one is absent the other must be present (of course they are both often reported). Example of an order set that includes terms with R-a conditionality:
| 24326-1 | Electrolytes 1998 panel | - | Pt | Ser/Plas | Qn | R/O/C |
|---|---|---|---|---|---|---|
| 2951-2 | Sodium | SCnc | Pt | Ser/Plas | Qn | R |
| 2823-3 | Potassium | SCnc | Pt | Ser/Plas | Qn | R |
| 2075-0 | Chloride | SCnc | Pt | Ser/Plas | Qn | R |
| 1963-8 | Bicarbonate | SCnc | Pt | Ser/Plas | Qn | R-a |
| 2028-9 | Carbon dioxide | SCnc | Pt | Ser/Plas | Qn | R-a |
| 33037-3 | Anion gap | SCnc | Pt | Ser/Plas | Qn | O |
The criteria or explanation for when the tests with R-a, Rflx and Rflx-a should be included in the results for a particular panel can be seen on the comprehensive details page available within RELMA and the online LOINC search application (search.loinc.org).
Note that the conditionality assigned to a term is panel-specific. In other words, a given test may be required in one context and optional in another, which is why both the conditionality and criterion for inclusion only appear in the panel details and not in the individual term details.
We should acknowledge that LOINC includes many panels without the required status having been marked and that the conditional status has not been employed everywhere that it could have been, and that many terms marked as conditional under the previous model probably would be better categorized as required with alternatives, reflex, or reflex with alternative. (We still have LOINC development work to do.)
7.4.1 Lupus anticoagulant testing as an example of complex conditionality
Here we describe lupus anticoagulant testing as a prototype to illustrate the use of conditionality. At the core of lupus anticoagulant testing is a three step process using a given coagulation measure, such as dRVVT. The first is a screening to determine whether a clotting abnormality exists. The second is a mixing study, in which the patient specimen is mixed with pooled normal plasma (PNP) and the same test (e.g. dRVVT) is rerun. This second test is used to evaluate whether the clotting problem is due to a clotting factor deficiency, such as occurs in Hemophilia. The third is yet another repeat of the same test (e.g. dRVVT) mixed with excess phospholipid – also called the confirmatory test. Lupus anticoagulant is declared present if the addition of excess phospholipid normalizes the result.
There are a number of variations in lupus anticoagulant testing algorithms across laboratories. Some labs do the confirm test second and the mixing study third (if needed). The International Society of Thrombosis and Haemostasis (ISTH) recommends using two clotting tests: dRVVT and aPTT-LA (lupus sensitive), each reflexing to confirm and/or mixing study. However, at least two labs use three screening tests, adding PT to the dRVVT and aPTT-LA, reflex to mixing and confirm studies from these, and add further reflex testing, including thrombin time and from that a reflex to reptilase time. There are also variations in how the results are reported, e.g. as actual clotting times and/or as ratios of the actual time to the time of normal. And some labs define the kind of phospholipid they use for the confirm study, i.e., hexagonal or platelet derived phospholipid.
The lupus anticoagulant panel for three screening tests shown below illustrates a number of issues related to conditionality described above. The three screening tests (dRVVT, aPTT and PT) are all required but can either be reported as the actual result or actual/normal, therefore all six terms are assigned the conditionality R-a. The mixing and confirmatory tests as well as thrombin time and reptilase time are only run if any of the screening tests are out of range and when run, can also be report as actual or actual/normal; therefore, all of these terms are assigned Rflx-a. The dRVVT and aPTT percent correction and screen to confirm ratio may not be reported by all laboratories and therefore have an O status. Finally, the overall interpretation must be reported and so is assigned R.
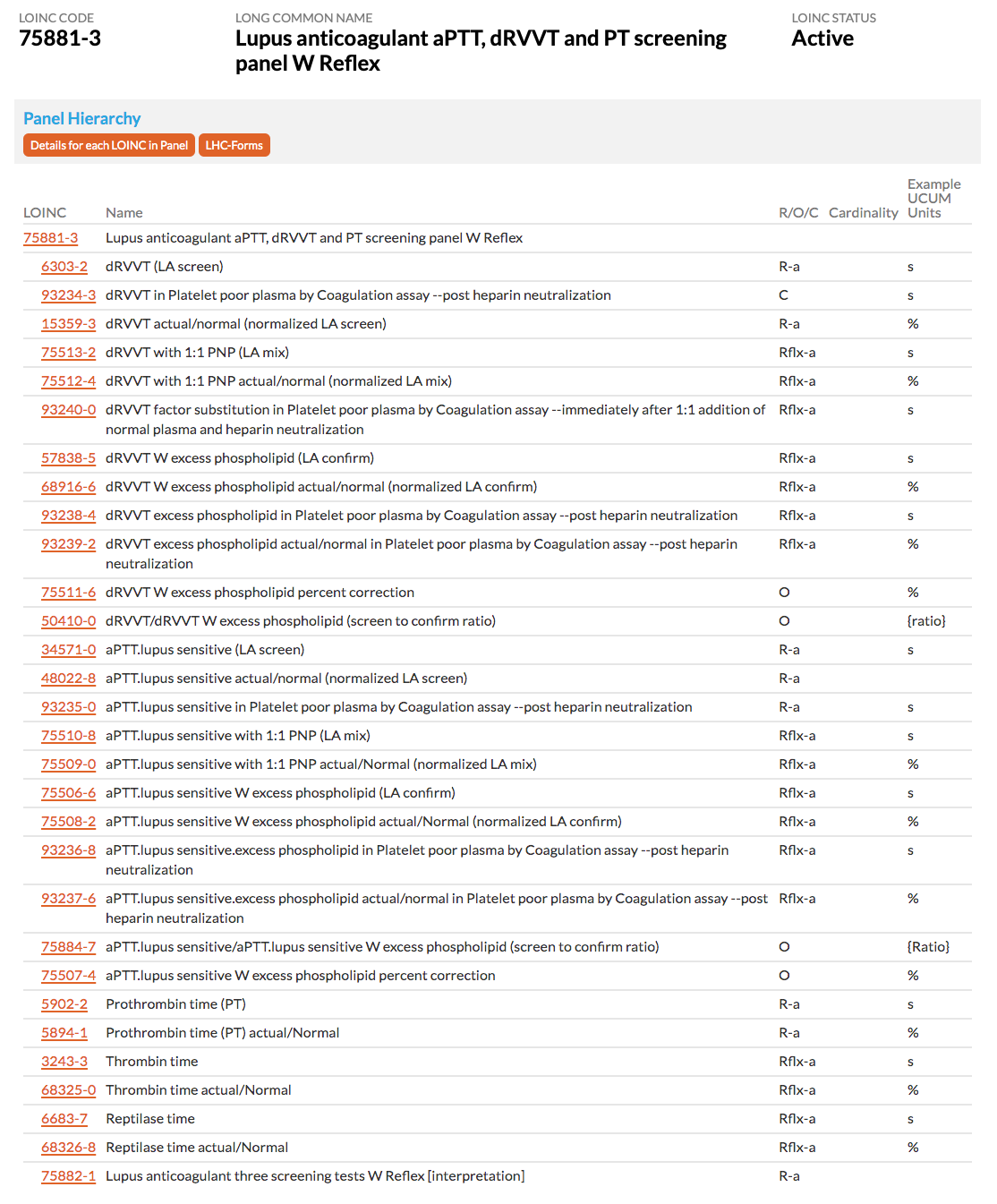
7.5 Approach to creating and defining distinct panels in LOINC
7.5.1 General approach
The defining characteristics of a LOINC panel term include the enumerated set of child elements and the conditionality of those elements. Thus, users must pay close attention to the full panel structure in order to know whether it is an appropriate representation of their local panel.
When LOINC defines a panel, it will include all of its primary measurements/observations (flagged as required), and will also include commonly reported derived observations (flagged as optional). When laboratories report an interpretation across many observations in a panel, such as the differential count or a serum electrophoresis, the LOINC panel will include an interpretation term specific to that panel (flagged as optional). Likewise, the panel will also contain commonly included AOE variables and other associated observations (flagged as optional).
LOINC will not define distinct panels for different collections that vary only on the optional elements (whether or not there is an interpretation, an additional derived calculation, variables sent along with the request, etc.). As a corollary, users can match their local panels to LOINC panels whether or not their reported results include those optional elements.
During the several years prior to the LOINC 2.56 release, the number of requests for panel terms steadily increased. At the same time, newer laboratory methods have led to the creation of lab kits that could be customized by the end-user in terms of the individual tests that were run. For example, if a kit includes ten different assays, one hospital might implement six while another hospital might implement all ten of the assays. In addition, each version of the kit could potentially have different combinations of tests. Many of our requests were for panels that differed only slightly from existing panels, likely in part due to differences in the aforementioned kit implementation. Going forward, it now appears impractical to create a unique panel for every hospital, laboratory, and test kit. Therefore, at the June 2016 meeting, the Laboratory LOINC Committee agreed upon the following business rules for new panel requests:
7.5.1.1 Panels for test kits
We will make generic panels for groups of tests that are not specific to a particular manufacturer or test kit but that are method- and system-specific. Such panels will not have conditionality or cardinality specified. As test kits evolve over time, additional children may be added, but none will be removed except in limited circumstances. Examples of such panels are:
79381-0 Gastrointestinal pathogens panel - Stool by Probe and target amplification method82180-1 Meningitis+Encephalitis pathogens DNA and RNA panel - Cerebral spinal fluid by Target amplification with non-probe based detection7.5.1.2 Panels for individual laboratories and/or hospitals
In general, we will not create new panels for every hospital and laboratory, based on their unique combination of elements. However, we likely will make panels when many hospitals and/or labs use the exact same set of elements.
7.5.1.3 Fully specified panels
We will make panels with conditionality and cardinality details in cases where a specific guideline or standard of care exists, such as the lupus anticoagulant screening panel examples above.
7.5.2 Preference for methodless panel definitions
In most cases, LOINC will not make up different panel terms for the same set of tests done by different methods. Because of the possible mixtures of methods within a panel, representing these distinctions would cause an explosion of the distinct panel, which would (usually) be a burden on the ordering provider. Further, in a given setting the ordering provider can only order the methods that are provided by his usual producer. Implied in the order is “Give me the battery produced by your usual methods”. Thus, as a general approach we tend to create panels with child elements that are methodless.
In special circumstances though, we do create panels of method-specific observations. For example, we have different panels for screen and confirm methods. In such cases, the method specification is the defining characteristic of the panel.
7.5.3 Special cases where enumeration of child elements is not practical
There are special cases where LOINC may define a panel term by a narrative definition because it is not practical to fully enumerate all of the possible child elements and their conditionality.
For example, public health, veterinary medicine, and other laboratories that are not charging Medicare or Medicaid for the tests they perform are not bound by the rules that penalize labs for performing and charging for tests that the practitioner did not explicitly order. This makes creating order panels a little easier.
In the case of public health, the tests performed by a laboratory in response to a request may be chosen from a list of many possible tests, based on information provided by the requester, and the laboratory’s judgment. For example, CDC’s “Campylobacter, Helicobacter and related organisms identification and subtyping” (CDC-10127) order can include tests that identify the phenotype, the genotype, Penner serotype, PFGE, or AST, and might use multiple test procedures for each of these.
Knowledge Base
- Home
- License
- LOINC Ontology Content Adjustments
- LOINC Release Notes
- Release Notes Archive
- LOINC Release Notes, February 2025 (Version 2.79)
- LOINC Release Notes, August 2024 (Version 2.78)
- LOINC Release Notes, February 2024 (Version 2.77)
- LOINC Release Notes, September 2023 (Version 2.76)
- LOINC Release Notes, August 2023 (Version 2.75)
- LOINC Release Notes, February 2023 (Version 2.74)
- LOINC Release Notes, August 2022 (Version 2.73)
- LOINC Release Notes, February 2022 (Version 2.72)
- LOINC Release Notes, August 2021 (Version 2.71)
- LOINC Release Notes, June 2021 (Version 2.70)
- LOINC Release Notes, December 2020 (Version 2.69)
- LOINC Release Notes, June 2020 (Version 2.68) & earlier
- Versioning
- Enriched Linkages between LOINC terms and LOINC Parts
- Abbreviations and acronyms used in LOINC
- Search API
Users’ Guide
- 1 – Introduction
- 2 – Major Parts of a LOINC term
- 3 – Special cases
- 4 – Clinical observations and measures
- 5 – Claims attachments
- 6 – Document Ontology
- 7 – Panels (Batteries)
- 8 – Evolving principles for naming collections
- 9 – Additional content in the LOINC distribution
- 10 – Standardized assessment measures
- 11 – Editorial policies and procedures
- 12 – Recommendations for best practices in using and mapping to LOINC
- A – LOINC Database Structure
- B – Classes
- C – Calculating Mod 10 Check Digits
- D – Procedure for Submitting Additions or Changes to LOINC
- E – Examples for LOINC Property Matching
- G – LOINC Technical Briefs
- D-Dimer Revisions in LOINC
- Choosing the Correct LOINC for Estimated Glomerular Filtration Rate
- Inducible Clindamycin Resistance in Staphylococcus and Streptococcus
- KIR Gene Family
- Oxygen Saturation and LOINC
- Nomenclature of Salmonella Species, Subspecies, and Serovars
- Segmented Neutrophils Versus Polymorphonuclear WBC
- Vitamin D
- Free Thyroxine Index Variants
- Streptococcus pneumoniae serotype nomenclature
- Non-linear Numerical Values “Binned” to Ordinal or Range
- H – LOINC Committee
- LOINC/RSNA Radiology Playbook User Guide
Search Syntax
RELMA
- Overview
- User Preferences
- Searching in RELMA
- Keyword Spell Check
- Term File Operations
- Import Local Terms
- Mapping Local Terms to LOINC
- HIPAA Claims Attachments
- Lab Auto Mapper
- Community Mapping Repository
- Requesting a new LOINC
